Mechanism of Battery-Induced Injury
©2010-2020 National Capital Poison Center. Reproduced and/or modified with permission.
Batteries cause tissue injury through three interacting mechanisms, although the relative contribution of each remains somewhat elusive. These mechanisms come into play when a battery is lodged in the gut, ear, nose or other orifice, rather than free-floating and in transit. The mechanisms, listed in the likely order of importance, include:
- Generation of an external electrolytic current that hydrolyzes tissue fluids and produces hydroxide at the battery’s negative pole,
- Leakage of battery contents, especially of an alkaline electrolyte, and
- Physical pressure on adjacent tissue.
Several authors have demonstrated that button battery-induced physical pressure or compression alone does not cause damage to the esophagus. Tanaka implanted and immobilized control batteries without battery contents (incapable of generating current or of leaking) in the esophagus of dogs, but found no damage to the esophageal mucosa or deeper tissues other than slight depression [1]. Yamashita implanted dummy batteries without contents and fully discharged batteries in the esophagus of 3 dogs and found flattening and compression without mucosal discoloration or erosion after 24 hours [2].
In vitro tests at pH 1.4, using hydrochloric acid to simulate the gastric environment, showed evidence of battery leakage as early as 2 hours after submersion (mean 26.5 hours, range 2-62 hours) with fully charged mercury cells but no leakage or corrosion when fully charged zinc air cells or discharged mercuric oxide cells were submerged in the same solution or in actual gastric fluid (pH 1.12) [3]. Since corrosion facilitates leakage by dissolving the battery can, and since a current is required for corrosion to occur, the absence of leakage from charged zinc air cells would be anticipated since no air was accessible to activate the cell. Similarly, discharged mercuric oxide cells would not generate an external current, although the authors failed to clarify whether these were fully discharged cells or cells discharged only to the level that would render them nonfunctional in products. The authors also failed to mention how leakage was determined.
The possibility of battery injury without leakage is supported by a number of investigations. In 1970, Leeming demonstrated alkaline dermal burns with pain and the most severe tissue damage at the negative electrode after applying low voltage DC current, just 3V, to the investigators hand. Litmus paper turned dark blue indicating a strong alkaline substance only at the burn site in contact with the negative electrode [4]. The disparity between the burns at the negative electrode compared to the positive electrode, and the buildup of alkali at the negative electrode suggest that the observed burns were not merely thermal. These findings are explained by the electrolysis of saline solutions (or tissue fluids), with sodium hydroxide and hydrogen gas generated at the negative electrode and chlorine gas, oxygen or both appearing at the positive electrode.
Yamashita inspected batteries removed from the nasal passages of two patients, one after 4 hours and the other after 2 days. Both patients had perforations of the nasal wall. However neither battery showed evidence that the battery contents had been disgorged. The authors surgically placed batteries in the upper esophagus of anesthetized dogs, lightly secured them in place with silk sutures to prevent movement, then examined the tissue pathology after sacrificing the dogs at intervals up to 72 hours. Tissue damage was evident, and ulceration seen by 4 hours, but there was no evidence that the batteries had leaked (no loss of potassium from the cells) until in the esophagus for more than 48 hours (one battery present for 72 hours leaked) [2,5]. Assuming that the necrotic tissue resulted from current flow rather than leakage (since there was no leakage up to 48 hours but there was tissue damage), the authors repeated the experiment, this time using a doubly-encapsulated battery (can within a can). Again tissue injury occurred despite the fact that there was no possibility of battery leakage. Finally, to exclude the role of physical pressure or compression causing tissue damage, the experiment was repeated with a completely discharged battery and a dummy battery (of the same shape and weight but with no alkaline contents); in both cases no tissue damage occurred. The authors further hypothesize that the damage was caused by the accumulation of sodium hydroxide at the negative electrode and this hypothesis was supported by the observation that tissue damage observed occurred in the section of the esophagus in contact with the negative pole (the term “anode” is used in this paper; see the note below about confusing terminology) but not sections in contact with the positive battery pole [cathode]. Based on the residual capacity of the 1.5 V, 220 mAh batteries used (59% after 48 hours), the authors estimate a discharge of 90.2 mAh. Since 1 Faraday (26.8 Ah) will generate 1 mol of NaOH, 134.6 mg of sodium hydroxide would have been generated – an amount sufficient to damage tissue if focused on one section of tissue rather than diluted and dispersed [5]. We add that this amount of sodium hydroxide is comparable to the total amount of potassium hydroxide found in a fully charged 1.5 V alkaline button cell (about 115 mg KOH), thus over time, the button cell is capable of generating more hydroxide through hydrolysis than it could release through leakage. The implication of the external current and electrolysis as the cause of injury suggests that lithium cells, often 3 V instead of 1.5 V, would be expected to be more dangerous than alkaline cells, even though lithium cells don’t contain an alkaline electrolyte which could leak. In addition, zinc-air cells would be expected to cause less damage as in much of the gut they would have limited access to the oxygen required for activation. Likewise, discharged cells would be expected to cause less damage – with the caveat that batteries discharged through use to the point that they no longer can power a product still contain significant residual voltage.
Yoshikawa confirmed these findings by implanting 12 mm diameter lithium cells (3V) and identical but fully discharged control batteries into the esophagus of rabbits [6]. The animals were sacrificed at intervals, and the pH of esophageal and tracheal surfaces, residual voltage and current were measured. Completely discharged batteries (to 0 V and 0 mA) implanted in the rabbit esophagus caused no histologic changes, corrosion, or battery leakage after 27 hours, confirming that pressure was not a major cause of injury. In contrast, the mucosal pH became acidic at the positive pole and alkaline at the negative pole, with significantly more severe injury on the alkaline side. A time-dependent decline in residual voltage and current was also observed, confirming that battery discharge through an external current had occurred.
Maves confirmed in cats that the orientation of the battery was an important predictor of injury, and also concluded that the negative pole was adjacent to the most severe area of the burn. However since his experiment involved alkaline and mercuric oxide cells (both with potassium hydroxide electrolyte that could leak), he attributed this finding to leakage at the weak point: the plastic seal [7].
These findings have clinical implications, suggesting that batteries positioned in the upper esophagus with an anteriorly-facing negative pole are at greater risk of tracheoesophageal (TE) fistula. Battery orientation is described in only a small number of reported cases, but where described, TE fistulas occurred when the negative pole faced the anterior esophageal wall [8,9,10].
ANODE vs. CATHODE – Beware the Confusing Terminology:
For non-rechargeable (primary) button batteries, the negative pole is termed the anode, the site where the external electrical current flows into the battery and electrons flow out; similarly, the positive pole is termed the cathode. However, when these same batteries are immersed in a saline solution and electrolysis occurs, the negative pole is redefined as the cathode, or the site of the reduction reaction (gain of electrons). Similarly, the positive pole of the battery becomes the anode, or the site of oxidation reactions (loss of electrons). To avoid confusion, the anode and cathode need to be associated with an electrochemical process. The terms change when the focus changes from the electrochemical process on the exterior of the battery (when the cell is immersed in an electrolyte) to the reaction on the interior of the can during normal cell discharge. During the exterior electrochemical process, the negative pole is cathodic; however that same negative can is called the anode when referring to the reaction on the interior of the can during normal battery discharge. As a result of the changing terminology, health professionals are urged to refer to the positive and negative pole of the button cell, since the terms anode and cathode reverse depending on the context of the discussion.
During electrolysis, the following reduction reaction (gain of electrons) occurs at the negative pole, leading to the accumulation of hydrogen gas and hydroxide ions at the negative pole:
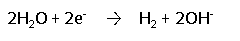
During electrolysis, the following oxidation reaction (loss of electrons) occurs at the positive pole, leading to the accumulation of chlorine gas at the positive pole:
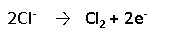
In vitro studies of hearing aid batteries (EP675E, 1.5V, mercuric oxide cells) immersed in a simulated gastric environment (0.1 N hydrochloric acid) showed significantly less crimp dissolution when cells were discharged prior to immersion. Likewise, less crimp dissolution was observed for discharged cells and dummy cells ingested by dogs [11]. Crimp dissolution serves both as a marker for leakage and for electrolysis. Leakage is facilitated by dissolution of the side of the battery can (the crimp), but that dissolution also requires corrosion caused by an external electrolytic current. Thus crimp dissolution fails to differentiate between the two injury mechanisms. Clinical data (human ingestion cases) fail to show a correlation between battery discharge state and patient outcome, likely because most outcomes are benign. However, focusing on the subset of large diameter cells, new cells were 3.2 times as likely to be associated with a moderate, major or fatal outcome compared to spent cells [12].
In 1986 Yasui reported studies of alkaline (manganese dioxide) 1.5 V, 11.6 mm diameter, button batteries implanted in the stomach or appendix of rats and in vitro investigations of batteries immersed in pH adjusted solutions [13]. This study provided definitive experimental confirmation of the role of an external electrolytic current. Through a series of in vivo and in vitro experiments, these authors demonstrated:
- The speed of electrolytic reactions was faster in acidic pHs compared to alkaline pHs, however over the range expected clinically, the differences were not profound. Electrolytic reactions were about 2/3 as great at a pH of 8 compared to a pH of 2.
- Prior to leakage of the alkali from the battery, there was a reduction in battery voltage, a rise in mucosal pH, and often ulceration or perforation. These effects occurred both in the acidic (gastric implant) and alkaline (appendiceal implant) models.
- Batteries discharged from 1.5 V to 1.2-1.3 V, the level at which electronic devices become nonfunctional, also showed continued discharge, electrolytic reactions, and intestinal perforation or necrosis when implanted in the rat appendix, demonstrating that even used batteries pose a danger on ingestion.
In 1998 Tanaka showed histopathological changes following fixation of the increasingly popular CR 2032 lithium batteries in the esophagus of dogs. No leakage of battery contents was observed in batteries removed 15 or 30 minutes after implantation, but necrosis had extended into the outer muscle layers of the esophagus. Leakage (determined by a reduction in residual amount of lithium perchlorate in the battery) was detected in batteries recovered 1 to 5 hours after implantation. However lithium batteries do not contain alkali and the leaking contents are not markedly irritating to tissues. Tanaka further demonstrated that sodium hydroxide is produced much more rapidly with lithium cells (3 V) than with other button cells (1.5 V) since the amount of alkali produced in tissue is proportional to the electric current produced, and the same amount of current is produced more rapidly with the higher voltage lithium cell [1].
Langkau used EPX 825 1.5 volt batteries constructed as a cell within a cell (primary cell contained in a second outer cell package for the purpose of achieving the correct physical battery size needed for a product). This doubly sealed construction makes rapid leakage extremely unlikely since only the outer can is exposed to fluid and susceptible to corrosion. A few drops of 1 N sodium chloride or tap water were placed on the negative terminal and a strip of pH-indicator paper laid across the terminal to bridge the insulating seal and provide an electrolytic path between the positive and negative terminals. A pH of 11 was reached in just 30 seconds with the sodium chloride solution and in 90 seconds with the less conductive tap water. These rapid pH changes suggest it is extremely unlikely that the change could be caused by leakage from the double sealed cell [14].
Rauber confirmed the alkaline pH change without leakage by extending electrodes from the battery poles and immersing only the electrodes, not the battery, in a compartmentalized beaker containing saline. An increase in pH was observed in just 15 minutes in the compartment with the negative electrode. Manganese dioxide 11.6 mm (A76) batteries were used in the investigation [15].
Experiments in rabbits showed significantly lower resistance (8 K Ohms) for artificial gastric fluid compared to stomach tissue (100-500 K Ohms), and concluded that most of the electrical current runs over the mucosal surface rather than through the tissue. A number of investigators have demonstrated considerable residual voltage (1.3 to 1.5 V) in button batteries which no longer powered the product [16].
Whether through leakage of an alkaline electrolyte or generation of an external current which then produces hydroxide, the caustic damage to the gastrointestinal mucosa can cause necrosis. While acid exposures cause a self-limiting coagulative necrosis, strongly alkaline substances produce a progressive liquefaction necrosis - solubilizing protein and collagen, saponifying lipids, and dehydrating cells, making perforation a more likely outcome.
Most severe complications following battery ingestions occur in the esophagus. Batteries must become lodged or impacted for tissue damage to occur. Batteries moving freely in the gut or surrounded by volumes of fluid do not cause focal tissue damage due to the failure of enough hydroxide to accumulate at one location to produce focal damage. The esophagus is especially susceptible to foreign body retention due to its several anatomic areas of narrowing and weak peristalsis.
Toby Litovitz, MD
National Capital Poison Center
________________________________________
REFERENCES
- Tanaka J, Yamashita M, Yamashita M. Esophageal electrochemical burns due to button type lithium batteries in dogs. Vet Hum Toxicol 1998;40(4):193-196.
- Yamashita M, Saito S, Koyama K, Hattori H, Ogata T. Esophageal electrochemical burn by button-type alkaline batteries in dogs. Vet Hum Toxicol 1987;29(3):226-230.
- Nolan M, Tucker I. Health risks following ingestion of mercury and zinc air batteries. Scand Audiol 1981;10(3):189-191.
- Leeming MN, Ray, Jr. C, Howland WS. Low-voltage, direct current burns. JAMA 1970;214:1681-1684.
- Yamashita M, Saito S, Koyama K, Noyama M, Hattori H, Naito S. Chemical burns due to low voltage direct current button type battery. (Translated from Japanese). Igaku no Ayumi 1983;126:957-959.
- Yoshikawa T, Asai S, Takekawa Y. Experimental investigation of battery-induced esophageal burn injury in rabbits. Critical Care Med 1997;25(12):2039-2044.
- Maves MD, Carithers JS, Birck HG. Esophageal burns secondary to disc battery ingestion. Ann Otol Rhinol Laryngol 1984;93(4 Pt 1):364-369.
- Sigalet D, Lees G. Tracheoesophageal injury secondary to disc battery ingestion. J Ped Surgery 1988;23(11):996-998.
- Grisel JJ, Richter GT, Casper KA, Thompson DM. Acquired tracheoesophageal fistula following disc-battery ingestion: can we watch and wait? Int J Pediatr Otorhinolaryngol 2008;72(5):699-706.
- Chiang MC, Chen YS. Tracheoesophageal fistula secondary to disc battery ingestion. Am J Otolaryngol 2000;21(5):333-336.
- Litovitz TL, Butterfield AB, Holloway RR. Button battery ingestion: Assessment of therapeutic modalities and battery discharge state. J Pediatr 1984;105:868-873.
- Litovitz T, Whitaker N, Clark L, White NC, Marsolek M: Emerging battery ingestion hazard: Clinical implications. Pediatrics 2010; 125(6):1168-1177. Epub 2010 May 24.
- Yasui T. Hazardous effects due to alkaline button battery ingestion: an experimental study. Ann Emerg Med 1986;15(8):901-906.
- Langkau JF, Noesges RA. Esophageal burns from battery ingestion. Am J Emerg Med 1985;3(3):265.
- Rauber A. Button batteries: letting the skeleton out of our closet. Vet Hum Toxicol 1990;32(5):460-464.
- Yamauchi K, Kobayashi T, Shinomiya T et al. Device for the removal of button batteries. Intern Med 2001;40:9-13.
